

PTC Supplier Directory | PTC Technology & Information Center | PTC Contract Research | Subscribe/Unsubscribe (below)
PTC Tip
of the Month - Catalyst
Separation
Sometimes we choose a phase-transfer catalyst more for its ability to be
separated easily from the product than for its reactivity (e.g., in
pharmaceutical applications in which product purity is more important than the
cost of using twice as much quaternary ammonium salt). For example, it is often
50 to 1000 times easier to wash methyl tributyl ammonium into water away from
the product than it is for the commonly used tetrabutyl ammonium (TBAB). This
results in lower aqueous waste as well as lower residual catalyst in the
product. On the other hand, if the product is water soluble (e.g., many
carboxylate salts), then we would want to choose a catalyst which distributes
99.9% or more into the organic phase in the presence of an aqueous phase with
high ionic strength. Such a catalyst may be methyl tricaprylyl ammonium.
Want to learn more about effective catalyst separation in real world PTC systems? Contact Marc Halpern at PTC Organics at tel +1 856-222-1146 or by E-mail.
Please forward this valuable PTC Tip of the Month to your colleagues and encourage them to subscribe
Free
PTC 2-Hour Seminar - October
2003 in New Jersey
PTC Organics will conduct a free 2-hour
PTC technical seminar entitled "Reducing Cost of Manufacture of Organic
Chemicals Using Phase-Transfer Catalysis" at its Mt. Laurel, New Jersey office in October 2003 (date
to be announced). Click here
for abstract (2 hours = seminar + Q&A). The meeting will include lunch and
will be held from noon to 2:00 pm for easy same day fly-in/fly-out (30 min from
Philadelphia airport, 60 min from Newark airport). If you are interested in attending, please contact
Marc Halpern at tel 856-222-1146 (US) or by E-mail.
PTC Reaction of the Month - Dehydrofluorination
US patent 6,548,719 (Honeywell) was issued just 3 weeks ago for a process for
producing fluoroolefins by PTC dehydrofluorination of HFC's. The inventors
demonstrate the effectiveness of crown ether. No
examples using quats are given in the patent, but the use of quats is discussed
and reads: "Among them [referring to quats],
benzyltriethylammonium chloride is preferred for use under strongly basic
conditions." Tip - That might not be
the "preferred" quat. Take the short quiz
below.
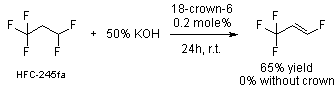
Quiz
(see answers at
bottom):
Question 1: Why did the inventors use crown ether and no examples with quats?
Question 2: Do you agree with the statement that benzyl triethyl ammonium
chloride (TEBA) is preferred as a quat under strongly basic conditions? Why do you think the inventors would say this?
Question 3: Based on the TEBA statement, do you think that the inventors took
our 2-Day PTC course?
PTC Breakthrough Process Screening
PTC Organics offers PTC
Breakthrough Process Screening for rapid and highly effective evaluation of breakthrough
phase-transfer catalysis processes. As the world leader in industrial
phase-transfer catalysis, PTC Organics develops
breakthrough high-performance low-cost PTC processes. Contact Marc
Halpern at PTC Organics by E-mail,
website, telephone (+1
856-222-1146) or fax (+1 856-222-1124).
Meet
PTC Organics at Chemspec
Learn how PTC Organics can help you improve process
performance and process R&D effectiveness using Phase-Transfer Catalysis by
scheduling a meeting with Marc Halpern at Chemspec to be held in
Manchester, UK on June 5, 2003. The meetings will be held in the SOCMA lounge
area (PTC Organics is a SOCMA member). Contact Marc
Halpern at PTC Organics by E-mail,
website, telephone (+1
856-222-1146) or fax (+1 856-222-1124).
Subscribe/Unsubscribe
To stop receiving the "PTC Tip of the Month," hit
"Reply" and type "Remove" in the Subject line.
To receive the "PTC Tip of the Month," Contact
Us and type "Subscribe to Tip" in the Comments.
Answers to Quiz
Answer 1. I don't have a clue. The very expensive crown ether wouldn't be my first choice (a quat would), especially at the low temperature, though to be fair, they did use only 0.2 mole%.
Answer 2. TEBA is almost never preferred for strongly basic PTC reactions (by PTC experts in the past 10 years) because it is just about the least stable quat under strong base conditions (3 ethyl groups are particularly susceptible to Hofmann elimination and the benzyl group is susceptible to nucleophilic attack). Moreover, TEBA generally provides lower reactivity than other quats in published comparative PTC dehydrohalogenations (because most PTC dehydrohalogenations are "I-reactions"). So why do people use TEBA so often for PTC/OH reactions? Because Makosza used it in hundreds of publications for strong base PTC reactions in the 1960's and 1970's and that's what you find when you do a literature search for PTC/OH. Want to learn more about choosing a catalyst for PTC dehydrohalogenation? Contact Marc Halpern at PTC Organics at tel +1 856-222-1146 or by E-mail.
Answer 3. No. As of the public course this week, > 500 chemists from >90 companies have participated in the 2-day course "Practical PTC" and they should all know the answer to Question #2 (we even do an exercise comparing TEBA to other quats for dehydrohalogenation). Has your company participated?
Before
performing any PTC reactions read this important message
Organic chemical reactions are inherently dangerous. Moreover, phase-transfer
catalysis may provide rate and other enhancements which can intensify associated
hazards. Under no circumstances should anyone perform any procedure on any scale
based in whole or in part on any of the contents of this E-mail before
thoroughly establishing safe operating procedures and performing a full and
competent hazardous operations analysis with the participation of qualified
technical personal trained in chemical, engineering, safety, industrial hygiene
and environmental disciplines and sciences.
Policy
The list of PTC Tip of the Month subscribers
will not be sold, rented or transferred and will be used only by PTC
Communications, Inc. and PTC Organics, Inc. to provide information related to
Phase-Transfer Catalysis. PTC Communications, Inc. reserves the right to
distribute the PTC Tip of the Month to selected industrial chemists, engineers and
managers.
PTC Tip of the Month #8 - May 2003
Copyright 2003 PTC Communications, Inc.